PTFE is marketed under the trade name of PTFE by DuPont It is a fully fluorinated thermoplastic having the following formula.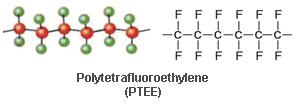
PTFE has an operating temperature range of from K208F to C4308C (K29Degree C to C212 Degrees C). This temperature range is based on the physical and mechanical properties of PTFE. When handling aggressive chemicals, reducing the upper-temperature limit may be necessary. PTFE is unique in its corrosion resistance properties. It is virtually inert in the presence of most materials. There are very few chemicals that will attack PTFE at normal use temperatures. Among materials that will attack PTFE are the most violent oxidizing and reducing agents known. Elemental sodium removes fluorine from the molecule. The other alkali metals (potassium, lithium, etc.) act in a similar manner. Fluorine and related compounds (e.g., chlorine trifluoride) are absorbed into PTFE resin to such a degree that the mixture becomes sensitive to a source of ignition such as impact. These potent oxidizers should be only handled with great care and recognition of the potential hazards. The handling of 80% sodium hydroxide, aluminum chloride, ammonia, and certain amines at high temperatures has the same effect as elemental sodium. A slow oxidation attack can be produced by 70% nitric acid under pressure at 4808F (2508C).PTFE has excellent weathering properties and is not degraded by UV light. Applications for PTFE extend from exotic space-age usages to molded parts and wire and cable insulation to consumer use as a coating for cookware. One of PTFE’s largest uses is for corrosion protection.
Including linings for tanks and piping. Applications in the automotive industry take advantage of the low surface friction and chemical stability using it in seals and rings for transmission and power steering systems and in seals for shafts, compressors, and shock absorbers.
Fluorinated ethylene-propylene is a fully fluorinated thermoplastic with Some branching, but it mainly consists of linear chains having the following Formula: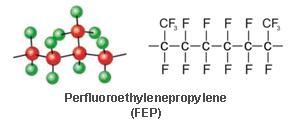
FEP has a maximum operating temperature of 375Degree F (190Degree C). After Prolonged exposure at 400Degree F (204C), it exhibits changes in physical strength. To improve some physical and mechanical properties, the polymer is filled with glass fibers.FEP basically exhibits the same corrosion resistance as PTFE with few exceptions but at lower operating temperatures. It is resistant to practically all chemicals except for extremely potent oxidizers such as chlorine trifluoride and related compounds. Some chemicals will attack FEP when present in high concentrations at or near the service temperature limit. FEP is not degraded by UV light, and it has excellent weathering resistance.
FEP finds extensive use as a lining material for process vessels and piping, laboratory ware, and other process equipment.
Perfluoralkoxy is a fully fluorinated polymer having the following formula.
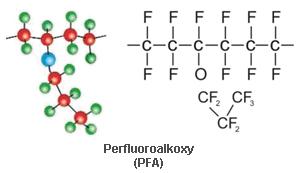
PFA lacks the physical strength of PTFE at elevated temperatures but has somewhat better physical and mechanical properties than FEP above 300Degree F (149Degree C) and can be used up to 500Degree F (260Degree C). Like PTFE, PFA is subject to permeation by certain gases and will absorb selected chemicals. Perfluoralkoxy also performs well at cryogenic temperatures.
PFA is inert to strong mineral acids, organic bases, inorganic oxidizers, Aromatics, some aliphatic hydrocarbons, alcohols, aldehydes, ketones, ethers, esters, chlorocarbons, fluorocarbons, and mixtures of these. Perfluoralkoxy will be attacked by certain halogenated complexes containing fluorine. This includes chlorine trifluoride, bromine trifluoride, iodine pentafluoride, and fluorine. It is also subject to attack by such metals as sodium or potassium, particularly in their molten states. Refer to PFA has excellent weatherability and is not subject to UV degradation.
PFA finds many applications in the chemical process industry for corrosion resistance. Applications includes lining for pipes and vessels.
Polyvinylidene fluoride is a crystalline, high molecular weight polymer containing 50% fluorine. It is similar in chemical structure to PTFE except that it is not fully fluorinated. The chemical structure is as follows:
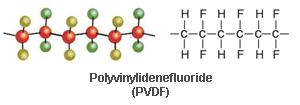
Much of the strength and chemical resistance of PVDF is maintained through an operating temperature range of K40 to C3208F (K40 to C1608C).
Approval has been granted by the Food and Drug Administration for Repeated use in contact with food in food handling and processing equipment. PVDF is chemically resistant to most acids, bases, and organic solvents. It is also resistant to wet or dry chlorine, bromine, and other halogens. It should not be used with strong alkalies, fuming acids, polar solvents, amines, ketones, and esters. When used with strong alkalies, it stress cracks.
PVDF also withstands UV light in the “visible” range and gamma radiations up to 100 Mrad.
Polyvinylidene fluoride finds many applications in the corrosion resistance field, being used as a lining material for vessels and piping, as solid piping, column packing, valving, pumps, and other processing equipment. Polyvinylidene fluoride is manufactured under the tradename of Kynarby Elf Atochem, Solef by Solvay, Hylar by Ausimont USA, and super Pro 230 and ISO by Asahi/America.
Polypropylene is one of the most common and versatile thermoplastics. It is closely related to polyethylene, both of which are members of a group known as polyolefins. The polyolefins are composed of only hydrogen and carbon.
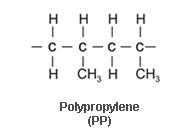
Polypropylene can be produced either as a homopolymer or as a copolymer with polyethylene. The homopolymers, being long chain high molecular weight molecules with a minimum of random orientations, have optimum chemical, thermal, and physical properties. For this reason, homopolymer material is preferred for difficult chemical, thermal, and physical conditions. Copolymer PP is less brittle than the homopolymers and is able to withstand impact forces down to K208F (K298C), whereas, the homopolymer is extremely brittle below 408F (48C).PP is not affected by most inorganic chemicals except the halogens and severe oxidizing conditions. It can be used with sulfur-bearing compounds, caustics, solvents, acids, and other organic chemicals. PP should not be used with oxidizing acids, detergents, low-boiling hydrocarbons, alcohols, aromatics, and some organic materials.
If exposed to sunlight, an ultraviolet absorber or screening agent should be in the formulation to protect it from degradation. Thermal oxidative degradation, particularly where copper is involved will pose a problem.
Polypropylene is widely used in engineering fabrics such as bale wraps, filter cloths, bags, ropes, and strapping. Piping and small tanks of polypropylene are widely used, and 90% of all battery casings are made of PP. Ignition resistant grades are available as a result of the addition of halogenated organic compounds. With this additive, polypropylene can be used in duct systems in the chemical industry. Because PP exhibits good flex life, it is useful in the construction of integral hinges. Polypropylene also has Many textile applications such as carpet face and backing yarn, upholstery fabrics, and diaper cover stock. It is useful in outdoor clothing and sports clothes that are worn next to the body because its wicking qualities absorb body moisture and still leaves the wearer dry. PP also finds application in the automotive industry for interior trim and under-the-hood components. In appliance areas, it is used in washer agitators and dishwasher components.
Consumer goods such as straws, toys and recreational items also make use of polypropylene.